Yeast produces rare drug: Genetically modified microbes produce valuable isoflavonoid from astragalus
Chinese researchers have developed a yeast platform that produces a valuable isoflavonoid from the medicinal plant Astragalus membranaceus. By reconstructing the entire biosynthetic pathway in Saccharomyces cerevisiae and systematically eliminating metabolic bottlenecks, the first yeast strain was created that produces the compound from simple carbon sources. Isoflavonoids such as formononetin, calycosine, and calycosine-7-glucoside are major constituents of Astragalus membranaceus, a plant widely used in traditional Chinese medicine. According to the researchers, these molecules have antioxidant, anti-inflammatory and heart-protective effects, which increases global demand. However, plant cultivation is slow, environmentally dependent and produces low yields, even with techniques such as hair rooting cultures or UV induction. In contrast, genetically modified microbes achieve high production volumes of related flavonoids in days instead of months. So far, the production of calycosin-7-glucoside in yeast has not been successful. Since the biosynthetic pathway from Daidzein is known, the compound is suitable as a test case for a microbial alternative to plant extraction. Due to these challenges, there is a need for a robust microbial production route.
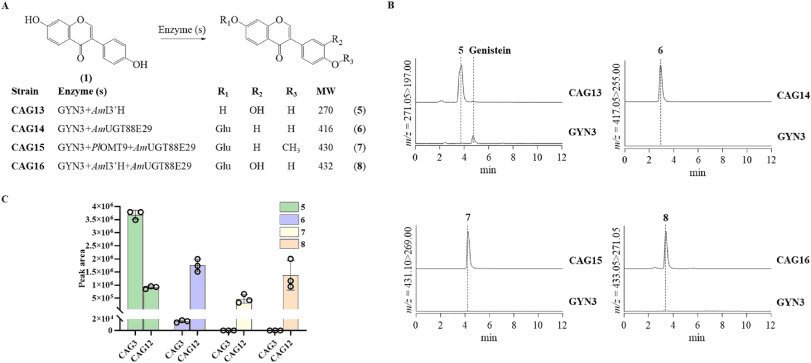
The study by the team led by Jiazhang Lian from Zhejiang University establishes a yeast-based platform for the de novo biosynthesis of calycosin-7-glucoside. This offers a scalable and efficient alternative to the plant-based production of high-priced isoflavonoids. Using step-by-step metabolic engineering and analytical strategy, the researchers reconstructed and optimized the de novo biosynthetic pathway in Saccharomyces cerevisiae. The starting point was a previously developed yeast strain that overproduces daidzein. Certain genes were integrated sequentially to build up the conversion cascade from daidzein to the target glucoside. Targeted gene knockouts, improvement of the precursor supply, enzyme replacement and optimization of the gene copy number eliminated metabolic bottlenecks. In parallel, LC-MS-based metabolomics was used, including selective ion monitoring, product ion fragmentation, and optimized MRM quantitation, to identify pathway intermediates and analyze metabolic flows in modified strains.
This integrated approach revealed that the introduction of a specific enzyme enabled formononetine formation, while subsequent expression of a hydroxylase intermediate rapidly converted to calycosine, suggesting high activity. However, the integration of the native calycosin-7′-O-glucosyltransferase only led to trace amounts of glucoside, which identified glycosylation as the primary bottleneck. Deletion of endogenous glucoside hydrolases, in particular of a specific gene, and an increase in the supply of UDP glucose moderately increased the product titers, but did not completely solve the bottleneck. The replacement with a more active glucosyltransferase dramatically shifted the metabolic flow to glycosylated products and increased the ratio of calycosine-7-glucoside to calycosine by over three orders of magnitude. Metabolite profiling showed that enzyme switching altered the abundance of several by-products, highlighting the importance of upstream checkpoints. Subsequent gene copy number optimization proved that increasing the dose of an early enzyme was most effective in increasing pathway flow, while additional copies of downstream enzymes provided limited benefit. The optimized strain achieved a final titer of 0.22 milligrams per liter of calycosine-7-glucoside within 48 hours, establishing an early enzyme as a central target and underscoring the need for advanced metabolic control strategies for further improvements.
This work thus creates a proof-of-concept platform for the production of calycosin-7-glucoside and related isoflavonoids in yeast. In the long term, such microbial systems could reduce dependence on medicinal plant cultivation, stabilize supply chains and reduce production costs for pharmaceutical and nutraceutical ingredients. Beyond this connection, the study provides general design principles ã combining metabolomics, enzyme screening, and gene copy optimization ã that are applicable to many other plant-based natural products. The method could revolutionize the industrial production of bioactive molecules and contribute to more sustainable alternatives in medicine and nutrition. Further optimizations, for example through advanced genetic engineering or fermentation conditions, promise higher yields and broader applications.
Original Paper:
Editor: X-Press Journalistenbû¥ro GbR
Gender Notice. The personal designations used in this text always refer equally to female, male and diverse persons. Double/triple naming and gendered designations are used for better readability. ected.




