Researchers clarify electron source for peroxiredoxin-6 enzymes
A research group at the Rhineland-Palatinate University of Technology (RPTU) has solved a biochemical mystery that has remained unsolved for more than 25 years. Peroxidases of the so-called peroxiredoxin-6 type, which break down hydrogen peroxide, obtain their electrons from hydrogen sulfide, the anion of hydrogen sulfide. The findings link peroxide metabolism with sulfide metabolism for the first time and have been published in the journal “Advanced Science”. The work was funded by the German Research Foundation.
The team led by biochemist Marcel Deponte studied enzymes from humans and the malaria pathogen Plasmodium falciparum. Both variants reacted very quickly with hydrogen sulfide and reduced hydrogen peroxide to water. This resulted in hydrogen disulfide, which is considered a possible source of protective persulfides. The results show that this mode of reaction probably occurs in many organisms with peroxiredoxin-6 enzymes.
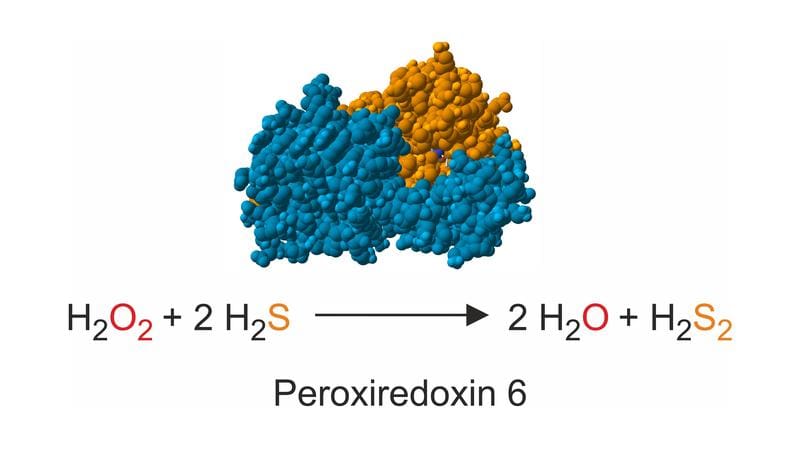
For the experiments, the researchers used the stopped flow method to record the particularly fast reaction sequences in the range of thousandths of a second. The enzymes were recombinantly produced and purified in E. coli bacteria. The easily comparable results from the two model organisms indicate a universal significance of these electron transfer mechanisms.
The research group has been working for years on redox enzymes and their role in the detoxification of hydrogen peroxide or their conversion into signaling processes. The current findings also contribute to the understanding of persulfide metabolism and expand the scientific basis for future work in molecular biology and biochemistry.
Original paper:
Editor: X-Press Journalistenb├╝ro GbR
Gender Notice. The personal designations used in this text always refer equally to female, male and diverse persons. Double/triple naming and gendered designations are used for better readability. ected.




