Protective mechanism enables cancer cells to survive under mechanical pressure
Researchers at the Cluster of Excellence CIBSS – Centre for Integrative Biological Signalling Studies at the University of Freiburg have discovered a protective mechanism that protects cancer cells from damage during metastasis. When migrating through narrow tissue structures, cancer cells are exposed to high mechanical pressure, which can cause the nuclear envelope to rupture. Normally, this would lead to DNA leakage and cell damage. However, the study shows that a fine framework of actin filaments stabilizes the cell nucleus within a few seconds and prevents DNA loss. This mechanism explains why cancer cells are able to continue their migration despite heavy stress. The results were published in the EMBO Journal.
High-performance microscopy reveals protective mechanism
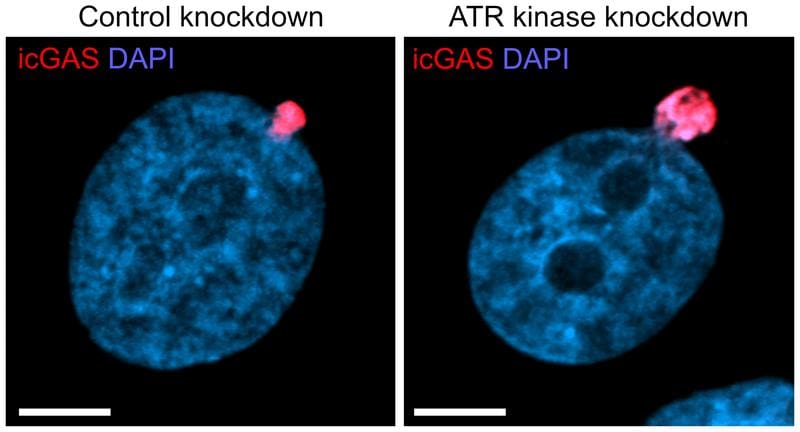
For the study, the scientists used a highly invasive cancer cell line (fibrosarcoma cells HT1080) and guided it through special microchannels with diameters of three or eight micrometers. Especially in the narrow channels, ruptures of the nuclear envelope occurred. About 80 seconds after such a rupture, actin filaments formed that stabilized the nucleus. With the help of atomic force microscopy, the mechanical stability of the cell nuclei was investigated. In collaboration with a colleague from the Cluster of Excellence CIBSS, it was possible to decipher the signal transmission of the DNA damage sensor ATR to the proteins DIAPH1 and DIAPH3, which stimulate the formation of actin filaments.
Key proteins for the stability of the cell nucleus
The study shows that the proteins DIAPH1, DIAPH3 and the DNA damage sensor ATR play a central role in the protective mechanism. If these proteins were blocked, the cell nucleus destabilized and DNA leakage increased. This finding opens up new perspectives for cancer research. In the long term, targeted interventions in this mechanism could be developed to prevent the formation of metastases or to treat diseases associated with unstable cell nuclei. Such approaches could form the basis for new therapeutic strategies.
Original Paper:
Editor: X-Press Journalistenb├╝ro GbR
Gender Notice. The personal designations used in this text always refer equally to female, male and diverse persons. Double/triple naming and gendered designations are used for better readability. ected.




