New molecule could revolutionize cancer therapies
Researchers at the University of Basel and the University Hospital Basel have developed a new fusion protein that could make immunotherapy against cancer more efficient and have fewer side effects. The molecule combines a variant of the signaling substance interleukin-2 (IL-2v) with an antibody that binds the protein PD-1 to immune cells in the tumor environment. The results were published in “Science Translational Medicine”.
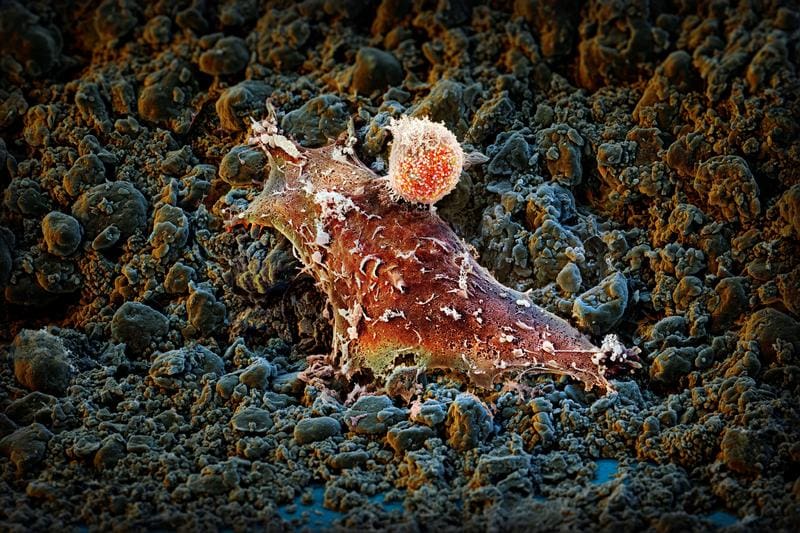
The fusion protein acts in two ways: the PD-1 antibody cancels out the “non-attack” signal that cancer cells use to inhibit the immune system, while IL-2v specifically activates immune cells that fight tumor cells. Unlike conventional IL-2, which established the first approved immunotherapy in 1984, the new molecule does not address the regulatory T cells that dampen the immune attack, but rather reactivates tumor-attacking immune cells, including exhausted cells.
Laboratory trials with cells from lung cancer patients confirmed the efficacy of the fusion protein, which was developed by the pharmaceutical company Roche and is currently being tested in a Phase I clinical trial. The researchers led by Prof. Dr. Alfred Zippelius see the potential for more precise cancer therapy with fewer side effects, which forms the basis for further optimization.
Original Paper:
Irene Fusi, Clara Serger, et al.
PD1-targeted cis-delivery of an IL-2 variant induces a multifaceted antitumoral T cell response in human lung cancer
Science Translational Medicine (2025), doi: 10.1126/scitranslmed.adr3718
Editor: X-Press Journalistenb├╝ro GbR
Gender Notice. The personal designations used in this text always refer equally to female, male and diverse persons. Double/triple naming and gendered designations are used for better readability. ected.




