New insight into bacterial infections caused by Yersinia enterocolitica
Researchers of Karlsruhe Institute of Technology (KIT) and the Max Planck Institute Marburg have gained new insights into the infection mechanisms of the bacterium Yersinia enterocolitica, which is related to the plague pathogen. The study, published in the journal PLOS Pathogens (DOI: 10.1371/journal.ppat.1013423), shows how the bacterium switches between replication and infection and opens up perspectives for new therapeutic approaches against bacterial resistance.
The bacterium Yersinia enterocolitica uses the type III secretion system (T3SS), a kind of molecular injection needle, to introduce disease-causing proteins into human cells and suppress the immune defense. However, this system has a disadvantage: while it is active, the bacterium stops its growth and cannot multiply. The researchers found that Yersinia enterocolitica solves this conflict through a molecular switch that regulates the activity of T3SS as a function of cell density.
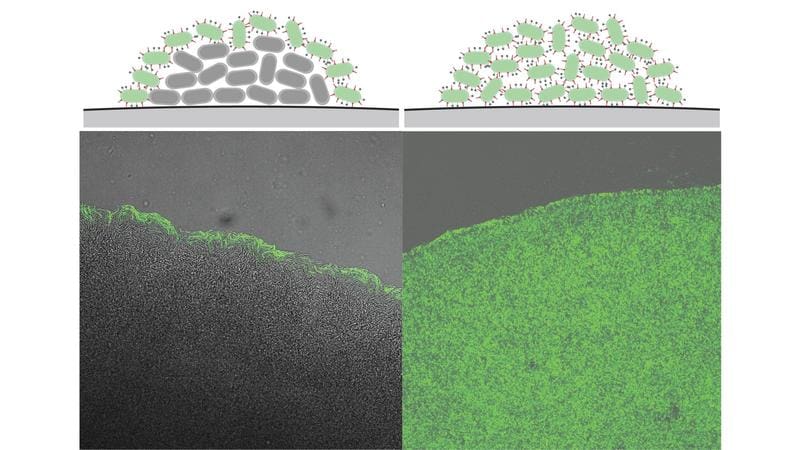
When there is a high bacterial density, the T3SS in the inner cells of a colony is deactivated so that they can divide further. Only the outer cells, which are exposed to the immune system, keep the secretory system active. This mechanism is controlled by the protein VirF, which is downregulated by small RNA molecules at high cell density. The shutdown is reversible, so the T3SS can be reactivated within 30 to 60 minutes if the bacteria spread.
In addition, the protein YadA, which is responsible for attaching to host cells, is deactivated at high cell density. This makes the bacterium more mobile and more difficult for the immune system to detect. This camouflage mode allows the pathogen to reach new tissues or resettle in the body.
The results illustrate that Yersinia enterocolitica actively switches between a virulent and a replicative state in order to both assert itself against the immune defense and to reproduce efficiently. This mechanism could explain why bacterial infections are often difficult to fight. The study emphasizes that the targeted shutdown of T3SS could be a potential target for new therapies. By understanding such switching mechanisms, approaches could be developed in the future that disrupt these processes and thus improve the treatment of bacterial infections, especially in view of growing antibiotic resistance.
Original Paper
Francesca Ermoli, Gabriele Malengo, Christoph Spahn, Corentin Brianceau, Timo Glatter and Andreas Diepold: Yersinia actively downregulates type III secretion and adhesion at higher cell densities, 2025 DOI: 10.1371/journal.ppat.1013423
Editor: X-Press Journalistenb├╝ro GbR
Gender Notice. The personal designations used in this text always refer equally to female, male and diverse persons. Double/triple naming and gendered designations are used for better readability. ected.




