Konstanz researchers decipher enzyme mechanism against cancer
An international research team led by biologists from the University of Konstanz has deciphered a molecular mechanism that regulates the activity of N-myristoyltransferases. These enzymes play a role in biological signaling pathways whose dysregulation can lead to the development of serious diseases such as cancer. The results of the study, published in the journal Molecular Cell, were obtained by scientists from the University of Konstanz together with colleagues from ETH Zurich and the California Institute of Technology.
Proteins are the basic building blocks of life and are continuously produced, transported and broken down in cells. Disruptions in these processes can lead to serious diseases. The researchers investigated the control of N-myristoyltransferases, enzymes that chemically modify proteins during their production and thus enable them to function. These modifications affect signaling pathways that play a role in cancer and viral diseases. The study sheds light on the mechanism by which these enzymes are activated at the output of the cellular protein factories, the ribosomes, and identifies a potential starting point for new drugs.
Protein production begins with the translation of genetic information into amino acid chains, followed by chemical modifications. Often the amino acid methionine is cleaved off, in some cases followed by the attachment of a fatty acid by N-myristoyltransferases. The team analyzed how these enzymes act on the ribosome and interact with competing enzymes.
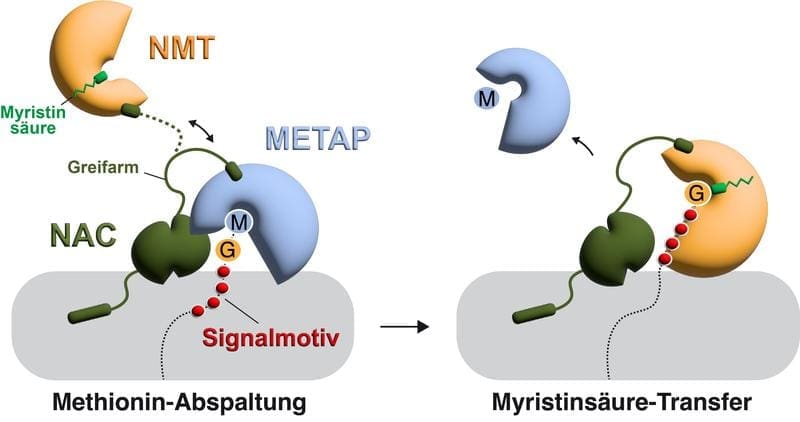
Using structural, quantitative and genetic methods as well as biochemical experiments, it was determined that the nascent polypeptide-associated complex plays a central role in coordination. This complex positions enzymes at the ribosomal tunnel where proteins are produced. As binding sites overlap, a controlled exchange takes place: first methionine cleavage, then fatty acid attachment, triggered by a signaling motif in the protein that is exposed after cleavage.
The nascent polypeptide-associated complex also regulates other enzymes that attach acetyl groups, a more frequent modification. The N-myristoyltransferases bind more tightly to the tunnel and thus gain a time advantage to act despite competition.
Deciphering the mechanism opens up new avenues for drugs against cancer and viral diseases. Current drugs inhibit the enzymes cell-wide and cause side effects. The study identifies the binding site between enzymes and the nascent polypeptide-associated complex as a selective starting point for more targeted therapies with potentially less toxicity.
Original Paper:
Mechanism of cotranslational protein N-myristoylation in human cells: Molecular Cell
Read also:
Liquid biopsy: Innovative test uses RNA to detect cancer at an early stage – MedLabPortal
Editorial office: X-Press Journalistenb├╝ro GbR
Gender note. The personal designations used in this text always refer equally to female, male and diverse persons. Double/triple references and gendered designations are avoided in favor of better readability.




