Human organoids: Gene therapy against blindness in childhood dementia successfully tested
Researchers at the University of T├╝bingen have demonstrated the efficacy of gene therapy against retinal damage in the rare childhood dementia CLN2-Batten syndrome in an innovative human laboratory model. Without animal experiments, it was possible to reduce disease-related processes in retinal organoids and a retina-on-chip system, which could prevent the progression of blindness in those affected. The results have already enabled the approval of a Phase I/II clinical trial in the UK, which is currently underway and is showing initial positive interim results.
Neuronal ceroid lipofuscinosis type 2 (CLN2) is caused by mutations in the TPP1 gene and leads to progressive degeneration of the brain and retina from infancy onwards. The photoreceptors responsible for vision degenerate rapidly, causing children to go blind within a few years. Without therapy, those affected rarely reach an age of more than eight to twelve years. Current treatments only partially slow down the neurological progression, but do not address the loss of vision. A team from the Institute of Neuroanatomy and Developmental Biology and the Institute of Biomedical Engineering simulated the disease in a realistic model: stem cell-based retinal organoids on a chip about 1 euro in size, which replicates the microenvironment of the retina, including an artificial blood flow. Here, the typical lipofuscin deposition could be precisely represented.
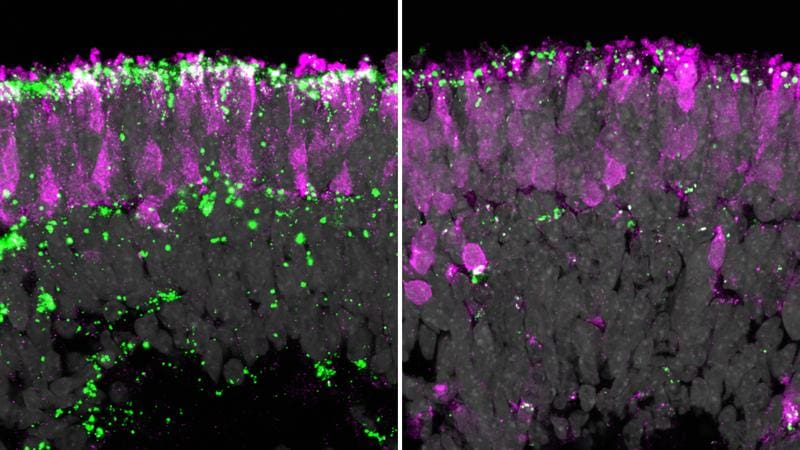
In this in vitro system, the scientists tested the gene therapy TTX-381 from the US company Tern Therapeutics. A modified adeno-associated virus (AAV) transports a functional copy of the TPP1 gene directly into the retinal cells to produce the missing enzyme. The treatment restored enzyme production, reduced harmful deposits or prevented them completely. This highlights the potential of therapy to stop blindness and demonstrates the reliability of animal-free models for preclinical testing.
Thanks to these data, the TTX-381-1102 study was approved at Great Ormond Street Hospital in London , without prior animal testing for efficacy testing. The Phase I/II dose-escalation study is recruiting children 1 to 12 years of age and has recently completed enrollment in the second cohort, with a dose selection for an extension cohort. Initial results indicate a stabilization or improvement in vision, which could significantly increase the quality of life. The study is registered with clinicaltrials.gov among NCT05791864 and is scheduled to end by mid or late 2025.
Tern Therapeutics, founded in 2023 with $15 million in seed funding, is developing further approaches against CLN2. In parallel to TTX-381, which is administered subretinally, TTX-181 is in preparation, a gene therapy for the brain via the cerebrospinal fluid pathway to slow neurological degradation and extend life expectancy. Both candidates come from a license from REGENXBIO and are aimed at a one-time treatment. Recently, Tern partnered with Andelyn Biosciences for late PPQ manufacturing of TTX-381 to accelerate scaling.
The cooperation between T├╝bingen and Tern marks a milestone: Retina organoids and organ-on-chip technologies enable human-specific simulations that complement or replace animal models and shorten development times for rare diseases. Around 400 million people worldwide suffer from such diseases, for which there are often no therapies. This method could change the standard in gene therapy research and lead more quickly to treatments for vulnerable patient groups.
Original Paper:
Editor: X-Press Journalistenb├╝ro GbR
Gender Notice. The personal designations used in this text always refer equally to female, male and diverse persons. Double/triple naming and gendered designations are used for better readability. ected.




