First high-resolution structure of proteasome storage granules deciphered
An international research team from the Max Planck Institute of Biochemistry, the University Medical Center G├Čttingen and the University of Toronto has for the first time visualized the molecular interior architecture of proteasome storage granules (PSGs) in intact cells. Using cryo-electron tomography, the scientists show that these membraneless organelles consist of fully assembled proteasomes in a paracrystalline, partially crystal-like arrangement. The structures keep the proteasomes in an inactive state and allow for rapid reactivation when energy supply is restored. The results were published in Cell.
Proteasomes are large, cylindrical protein complexes that act as energy-dependent “protein shredders” in all eukaryotic cells. They break down marked, damaged or superfluous proteins into small peptides and consume ATP in the process. In the event of a lack of nutrients or energy ŌĆō for example due to glucose deprivation or blockade of mitochondrial ATP production ŌĆō yeast cells assemble their proteasomes into membraneless proteasome storage granules. Until now, PSGs have only been visible as fuzzy droplets under the fluorescence microscope.
Cryo-electron tomography with a resolution of 0.9 nanometers made it possible for the first time to study the PSGs in their natural cellular environment. The images show that the proteasomes first assemble to form trimeres ŌĆō a structure that was previously unknown in cells. These trimers stack up to form fibers, which in turn organize into bundles. The arrangement is locally highly ordered and paracrystalline, but does not have a perfect distant order as in real crystals. As a result, the proteasomes remain completely intact but inactive and do not consume energy.
“The structures decay as soon as you try to isolate the PSGs,” explains co-first author Dr. Lu Qu from the MPI of Biochemistry and the University Medical Center G├Čttingen. “Only cryo-electron tomography in intact cells has allowed us to see this precise organization.” When glucose is administered, the granules dissolve within an hour, and the proteasomes return to their functional state.
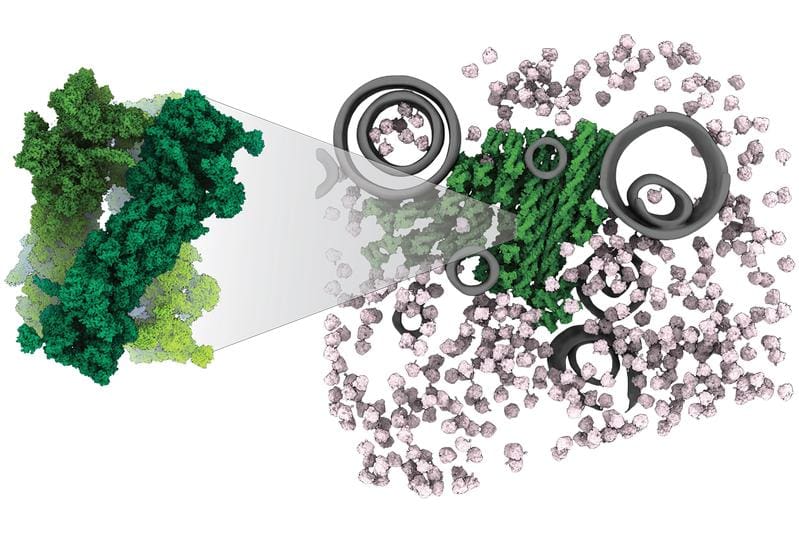
The discovery represents a new paradigm for membraneless organelles. Unlike the classic liquid-like droplets (e.g., stress granules), PSGs are based on specific, repetitive protein-protein interactions between structured molecular machines. “This arrangement allows the cell to keep energy-intensive proteasomes safely in reserve and to reactivate them immediately when needed,” says Prof. Brenda Schulman, Director at the MPI of Biochemistry.
The results have significance beyond basic research. Proteasomes play a central role in the effect of proteasome inhibitors used in cancer therapy. A better understanding of their regulation under stress could open up new approaches for the treatment of tumor cells or neurodegenerative diseases in which protein degradation is disrupted.
The study was carried out in close cooperation between the research groups of Prof. Brenda Schulman (MPI of Biochemistry), Prof. Wolfgang Baumeister (pioneer of cryo-electron tomography), Dr. Cordula Enenkel and Prof. Oliver Ernst (University of Toronto). First authors are Dr. Xiaomeng Tang and Dr. Lu Qu.
Original Paper:
Xiaomeng Tang, Lu Qu, Florian Wilfling, Florian Beck, Oliver P. Ernst, Brenda A. Schulman, Wolfgang Baumeister, Cordula Enenkel: Metabolically regulated proteasome supramolecular organization in situ, Cell, January 2026
Editor: X-Press Journalistenb├╝ro GbR
Gender Notice. The personal designations used in this text always refer equally to female, male and diverse persons. Double/triple naming and gendered designations are used for better readability. ected.




