Central mechanism of protein folding decoded
Researchers at the Center for Medical Biotechnology (ZMB) at the University of Duisburg-Essen, in collaboration with national partners, have decoded a central mechanism of protein folding in cells. Errors in this process can trigger serious diseases such as neurodegenerative disorders. The study focuses on the BiP-GRP94 chaperone complex, which plays a crucial role in the correct folding of proteins in the endoplasmic reticulum, the production and control center of the cell. The results were published in the journal Nature Structural & Molecular Biology.
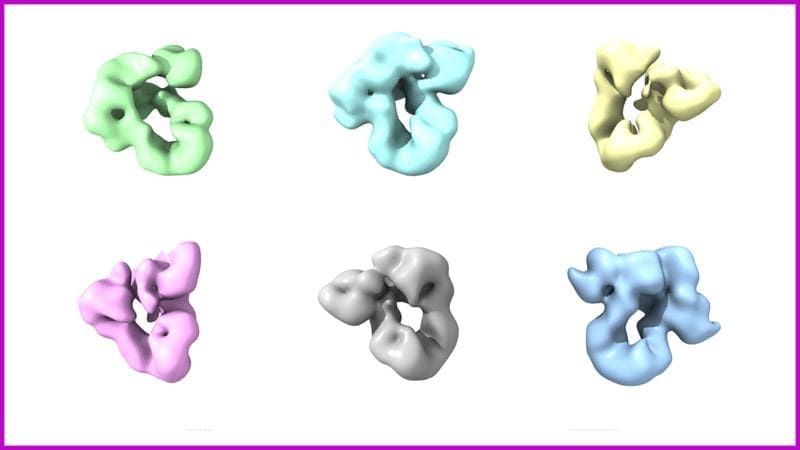
The study shows for the first time at a structural level how the two chaperones BiP and GRP94 work together. Using high-resolution electron microscopy and biochemical analyses, the scientists were able to visualize previously unknown conformations of the complex and decipher their function. The flexible structure of the complex makes it possible to support the folding of a large number of proteins.
The results provide important insights into the molecular machinery of the cell and could contribute in the long term to the development of new therapies for diseases with disturbed protein folding. The research was carried out as part of the DFG-funded Collaborative Research Center 1430 and underlines the importance of interdisciplinary cooperation at the University of Duisburg-Essen and with partners.
Original Paper:
Conformational plasticity of a BiP-GRP94 chaperone complex | Nature Structural & Molecular Biology
Editorial office: X-Press Journalistenbû¥ro GbR
Gender note. The personal designations used in this text always refer equally to female, male and diverse persons. Double/triple references and gendered designations are avoided in favor of better readability.




