Cell-based screening: New method allows identification of molecular adhesives
Researchers from the CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences, the AITHYRA Research Institute for Biomedical Artificial Intelligence and the Scripps Research Institute have developed a systematic method to identify molecular adhesives on a large scale. These active ingredients redirect the cell’s own protein degradation system specifically to disease-causing proteins that are difficult to attack and lead to their complete removal from the cell.
Many diseases are caused by proteins that can hardly or not at all be inhibited with conventional drugs. Molecular adhesives offer an alternative approach here: they induce new interactions between a target protein and a degradation enzyme, whereby the protein is ubiquitinated and degraded in the proteasome. So far, the discovery of such adhesives has mostly been accidental.
The new strategy combines high-throughput chemistry with cell-based screening. Starting with a small molecule that already binds to a target protein, the scientists generated thousands of chemical variants by systematically attaching different building blocks. These variants slightly change the protein surface and potentially enable new interactions. The compounds were tested directly in living cells without prior purification, with a sensitive assay detecting the degradation of the target protein.
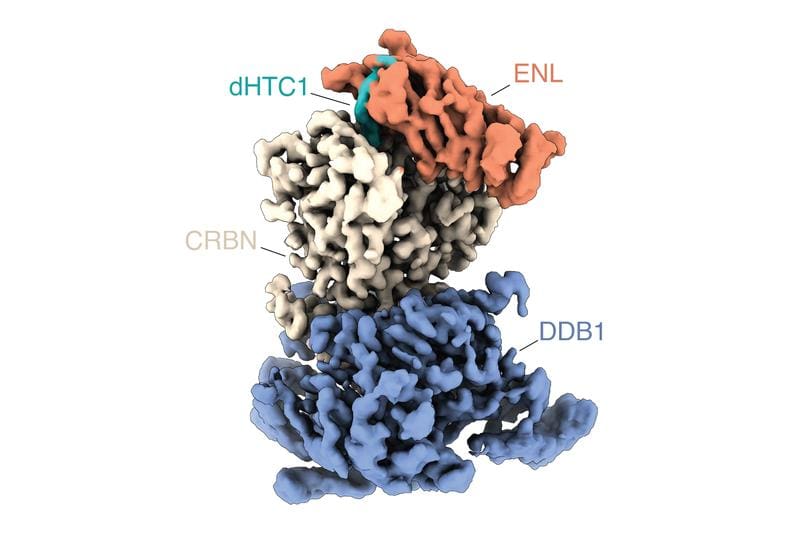
As a proof-of-concept, the researchers focused on the protein ENL, which plays a central role in certain aggressive forms of acute leukemia. From several thousand compounds tested, they identified a molecule that efficiently and selectively degrades ENL in leukemia cells. Further investigations showed that the compound primarily influences ENL and the gene programs it regulates, which strongly inhibits the growth of ENL-dependent leukemia cells. The mechanism of action is cooperative: the molecule first binds to ENL and then creates a new interaction surface that recruits a ubiquitin ligase and marks ENL for degradation. This mechanism ensures high efficacy and selectivity.
The method, which is based on sulfur(VI) fluoride exchange-based high-throughput chemistry, is broadly applicable and transforms the discovery of molecular adhesives from a randomly based to a rational and scalable process. In the long term, this could lead to new therapeutic options for proteins that were previously considered invulnerable.
The study was led by Georg Winter (AITHYRA and CeMM) and Michael Erb (Scripps Research Institute). It has been published in the journal Nature Chemical Biology (DOI: 10.1038/s41589-025-02137-2).
Original Paper:
Editor: X-Press Journalistenb├╝ro GbR
Gender Notice. The personal designations used in this text always refer equally to female, male and diverse persons. Double/triple naming and gendered designations are used for better readability. ected.




