Calcium-sensitive switch increases the effectiveness of cancer drugs
Antibody drugs to fight cancer are supposed to penetrate tumor cells and release a deadly charge deep inside, but all too often they don’t make it that far. A new study shows how this Trojan horse strategy works better by exploiting calcium differences outside and inside cells.
A research team led by Sophia Hober, a professor at the KTH Royal Institute of Technology, reported developing a calcium-activated delivery system that they believe could allow for more precise treatment with lower doses and less collateral damage to healthy tissue. In collaboration with Stanford University and UmeûË University, the researchers published their findings in PNAS, the journal of the National Academy of Sciences.
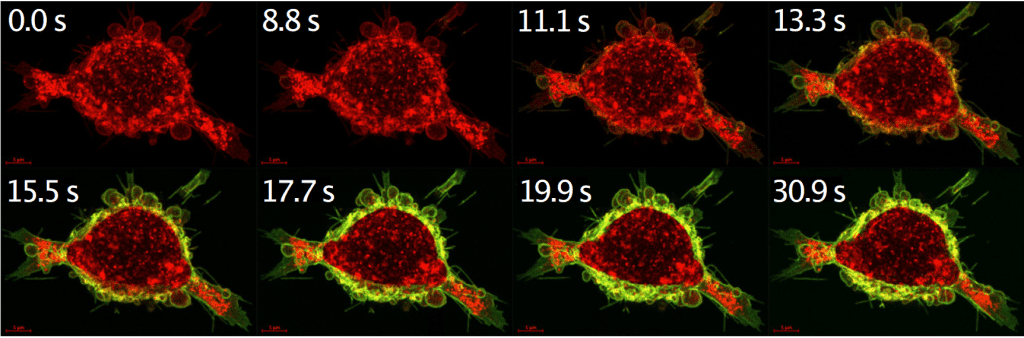
The concept targets a common challenge in targeted drugs that tend to bind too strongly to the receptors expressed by tumors. On the positive side, this strong binding blocks the tumor growth signals of the receptors. But ADCs (antibody-drug conjugates) are also designed to attack and kill, and all too often the protein can get stuck without ever penetrating deeper into the cell’s actual target: an acidic compartment called a lysosome. There, in the kill zone, the target protein can be degraded, releasing a toxin that causes cell death.
To avoid this problem, the researchers developed a calcium-sensitive switch that binds tightly to the cancer cell receptor on the outside of the cell, where relatively high concentrations of calcium are found in the blood and extracellular fluid.
Once connected, the drug-laden protein (or calcium-regulated affinity, CaRA) and epidermal growth factor receptor (EGFR) are drawn into the cell, into compartments with gradually decreasing calcium levels. And since their connection is calcium-dependent, the receptor and CaRA eventually go their separate ways: the receptor can return to the membrane, while CaRA transports its charge further to the lysosome.
“The calcium switch is integrated into the drug design,” says Hober. “It measures the calcium level and automatically adjusts its binding force.”
Original Paper:
PNAS,
DOI: 10.1073/pnas.2509081122
Editor: X-Press Journalistenbû¥ro GbR
Gender Notice. The personal designations used in this text always refer equally to female, male and diverse persons. Double/triple naming and gendered designations are used for better readability. ected.




