New biomarkers could enable earlier diagnosis of osteoarthritis
Researchers have used advanced spatial mass spectrometry imaging (MALDI-MSI) and proteomics of synovial fluid to demonstrate early molecular remodeling processes in subchondral bone in knee osteoarthritis (OA). These changes are already occurring under intact cartilage and could serve as biomarkers for earlier diagnosis and better monitoring of disease progression.
Osteoarthritis affects over 500 million people worldwide and is one of the leading causes of pain, disability and reduced quality of life. The disease is usually only diagnosed when cartilage loss is radiologically detectable and symptoms are present ŌĆō by which time the disease is already well advanced. Cartilage damage is largely irreversible, which makes early intervention difficult.
The study, published on January 26, 2026 in the journal Bone Research (Volume 14, Item No. 14, DOI: 10.1038/s41413-025-00495-0), examined human knee joint tissue from patients with end-stage OA and non-OA controls. Enzymatic targeting of extracellular matrix proteins and high-resolution spatial mapping made it possible to distinguish cartilage and bone molecularly.
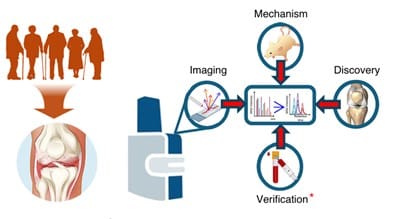
Under damaged cartilage, the subchondral bone showed strong upregulation of specific collagen fragments and post-translational modifications associated with tissue stiffening and remodeling. Similar signatures also occurred under structurally intact cartilage ŌĆō an indication that bone-related changes begin earlier than previously thought.
Many of the identified protein fragments from the bone were detectable in the synovial fluid. In contrast, traditional cartilage markers were reduced in OA synovial fluid. This makes bone-specific signatures a promising source of minimally invasive biomarkers.
The study was led by the Buck Institute for Research on Aging , led by Prof. Birgit Schilling (Executive Director of the Proteomics and Metabolomics Core) with significant contributions from Dr. Charles A. Schurman (postdoctoral researcher) and Dr. Joanna Bons (research associate).
The findings change the understanding of OA as a disease of the entire joint, not just cartilage. Changes in osteoblasts, osteoclasts and osteocytes in subchondral bone influence cartilage health through mechanical and biochemical signals. Future studies will further clarify these processes in animal models.
The findings provide the basis for fluid-based tests for the early detection of at-risk patients, monitoring the course and developing targeted therapies that could slow or prevent progression before irreversible damage occurs.
Original Paper:
Editor: X-Press Journalistenb├╝ro GbR
Gender Notice. The personal designations used in this text always refer equally to female, male and diverse persons. Double/triple naming and gendered designations are used for better readability. ected.




