Breakthrough in Alzheimer’s research: Structure of clusterin deciphered
A research team from the Max Planck Institute of Biochemistry has elucidated the three-dimensional crystal structure of the human protein clusterin for the first time using X-ray crystallography. The study shows that clusterin consists of three domains, with two disordered, hydrophobic peptide processes being crucial for its multiple protective functions. These processes allow clusterin to bind misfolded proteins such as amyloid-beta, tau and ╬▒-synuclein, which are associated with neurodegenerative diseases such as Alzheimer’s and Parkinson’s, and prevent their harmful aggregation in the extracellular space.
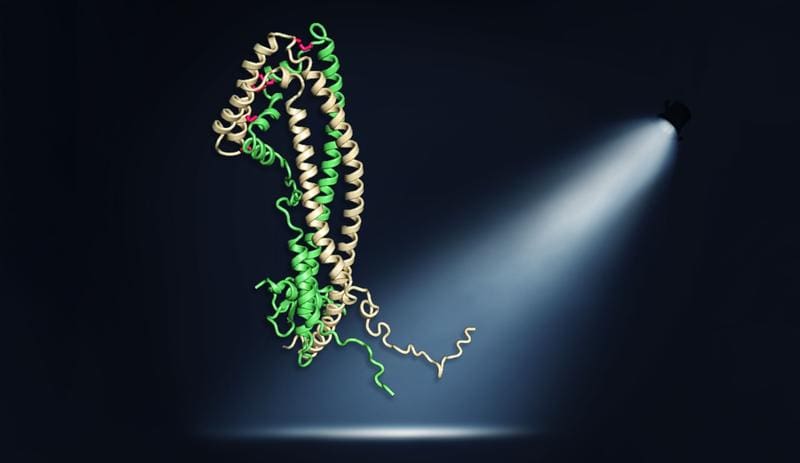
The study shows that the chaperone function of clusterin, which inhibits protein clumping, is based on these hydrophobic peptide processes. Biotechnological modification or removal of the hydrophobic amino acids in these processes eliminated the protective function against amyloid-beta aggregation. The peptide processes are also responsible for binding to cell surface receptors and the formation of lipoprotein complexes.
The results are of high medical relevance. Clusterin, also known as apolipoprotein-J, shows elevated levels in the cerebrospinal fluid of Alzheimer’s patients and binds extracellular amyloid beta plaques. The new findings on the structure and function of the protein provide important approaches for the research and development of new therapies against neurodegenerative diseases.
Original Paper:
Read Also:
Green light for new Alzheimer’s drug – MedLabPortal
Editor: X-Press Journalistenb├╝ro GbR
Gender Notice. The personal designations used in this text always refer equally to female, male and diverse persons. Double/triple naming and gendered designations are used for better readability. ected.




