New support for people with retinal diseases: Temedica and Roche launch digital companion “Retinora”
Roche Pharma AG and the Munich-based health insights company Temedica announce the launch of the digital patient companion Retinora as part of a close and long-standing cooperation.
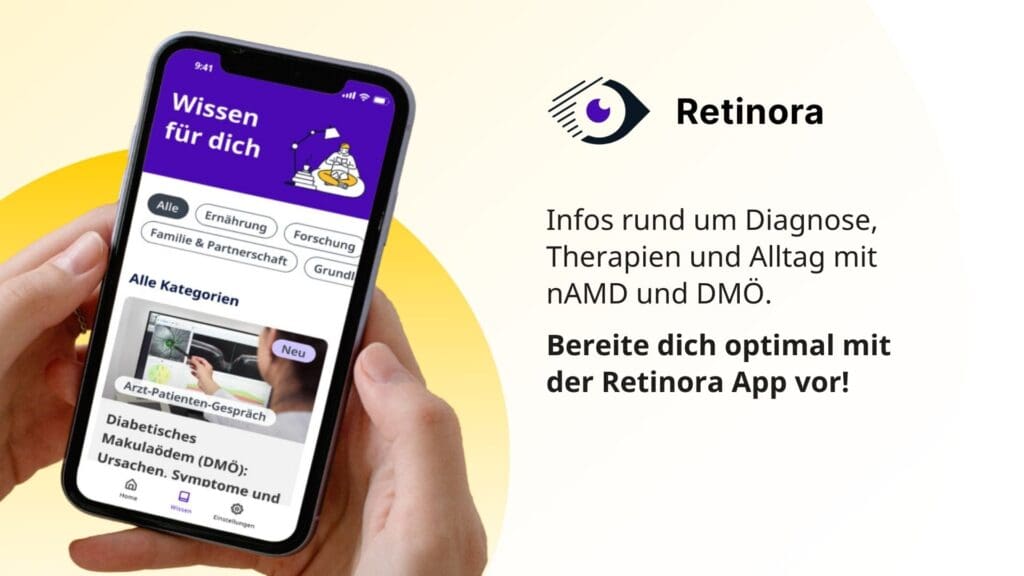
The Retinora digital companion app was developed specifically for people with wet age-related macular degeneration (nAMD) and diabetic macular edema (DME) and is the result of intensive collaboration with patients and technology experts.
Diabetic macular oedema (DME) is an accumulation of vascular fluid in the retina that occurs as a result of diabetes mellitus and can lead to vision loss or even blindness. Age-related macular degeneration (nAMD) also causes damage to the retina in the area of the macula. This significantly impairs the ability to see clearly and cannot be compensated for by wearing glasses.
Retinora integrates a wide range of functions that are tailored to the individual needs and challenges of patients with eye diseases. These include optimized appointment organization with a reminder function and comprehensive information on retinal diseases to keep users up to date on the latest diagnosis and treatment methods. In the future, the app will also integrate text-to-speech functions, color recognition and other specialized features to promote the independence of those affected in everyday life.
Through the connection to Temedica’s real-world evidence platform “Permea”, users regularly receive valuable insights generated from current research results and the aggregation and analysis of diverse data sources. In addition, app users have the opportunity to share their experiences for scientific research, actively contributing to improving the care of patients with wet AMD and DME. All data is used in strict compliance with the General Data Protection Regulation (GDPR) and with the express consent of the users.
With the development of Retinora, Roche and Temedica underline their leading role in the digital healthcare landscape and set a strong signal for growth and innovation in this sector. Retinora marks a significant step forward in promoting the independence and well-being of patients with nAMD and DME.
About Roche in Germany
Roche employs around 18,250 people in Germany in the areas of pharmaceuticals and diagnostics. The company is represented at three major sites in Grenzach-Wyhlen (Roche Pharma AG), Mannheim (Roche Diagnostics GmbH, Roche Diagnostics Deutschland GmbH, Roche Diabetes Care GmbH and Roche Diabetes Care Deutschland GmbH) and Penzberg (Biotechnology Center, Roche Diagnostics GmbH). The focal points cover the entire value chain of the two business areas “Pharmaceuticals” and “Diagnostics”: from research and development to production, logistics, marketing and sales, whereby each location also performs global tasks in addition to the German business. Roche is clearly committed to its German sites and has invested more than 5.5 billion euros in them over the past ten years. Roche has invested 13.2 billion Swiss francs in research and development globally in 2023. Further information on Roche in Germany at www.roche.de.
About Temedica GmbH
Temedica is a health insights company based in Munich. Since 2016, Temedica has been operating Europe’s leading ecosystem for Real World Insights in the healthcare sector. Temedica’s mission is to enable personalized and individualized medicine and thus focus on patients and their individual needs. The company achieves this by linking health-related data from various sources, which is processed into valuable insights in compliance with the GDPR.
Temedica is backed by a consortium of renowned investors with many years of experience in the biopharmaceutical industry, including the founding investors of BioNTech. Further information can be found at https://temedica.com/de/.
The respective companies are responsible for the content of the “Corporate News” section.